
Patients have become wary of India-made drugs after a number of reports highlighted their substandard quality. But regulating the over 20,000 domestic pharmaceutical companies remains a serious challenge.
An otherwise cheerful wholesale trader of generic medicines, Amrit Pal Gupta, who also owns a pharmacy in a posh locality in Delhi's Lodhi Colony, gets agitated these days whenever there is a mention of the quality of the products he sells.
Gupta, who has been promoting low-priced, India-made generic medicines for the past two decades, has had to field a growing number of queries concerning generic drugs. His sales have also taken a hit in the last two years.
"Can you cite even a single example or evidence of a generic medicine that has done any harm to any patient?" Gupta instantly counters when asked about the safety of medicines manufactured in India. Instead, he shuns multinational pharmaceutical companies for promoting their expensive brands through "mean strategies".
…
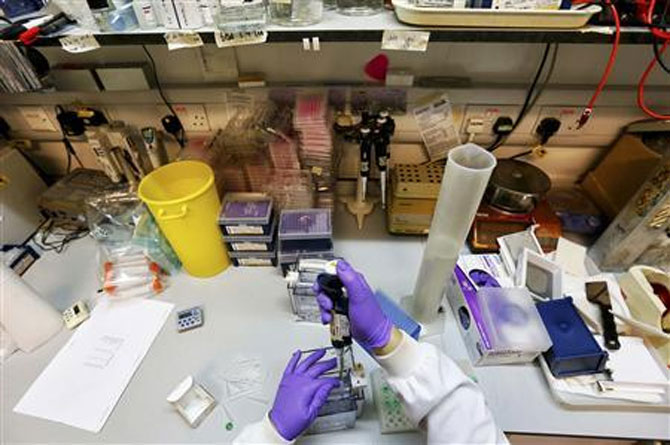
Like Gupta, of late, many other chemists, doctors and executives working with pharmaceutical companies are confronted with questions related to quality, safety and efficacy of Indian medicines.
"Patients are certainly much more curious and inquisitive these days," says a senior medical practitioner at IndraprasthaApolloHospital, Delhi. "The negative publicity that this industry has received in the last one year has done serious damage to its credibility and the trust of patients has been impacted. It will take years to restore its reputation," says the doctor. Some patients, he adds, simply refuse to accept prescriptions for generic medicines and insist on imported brands.
India-made medicines, which are much cheaper than imported drugs and have, therefore, been the lifeline for various developing countries so far, have come under the regulatory lens, more so in the past one year.
…

Ranbaxy, which gained much negative publicity after pleading guilty in America to fraud and admitting to charges of producing fake data to get fast approvals for generic medicines, hurt the industry the most. Faced with criminal charges, the company had to pay a fine of $500 million to US authorities.
But this was just the beginning of the troubles for Ranbaxy and for others in the business.
While more Ranbaxy facilities were proscribed by the US, other leading generic players such as Wockhardt, Agila Specialities, RPG Life Sciences and a facility of Sun Pharma too came under the US Food and Drug Administration's scanner.
Many batches of the medicines were recalled from America, the world's largest pharmaceutical market. Some factories of Ranbaxy and Wockhardt also faced strict scrutiny in Europe.
The FDA's observations during the inspections of Indian facilities brought to light some dirty facts. The findings of these observations, unlike those of other inspections, were subsequently made public.
…

The FDA's inspection of Ranbaxy's Mohali factory had, for instance, revealed the presence of hair and grease spots in tablets.
The factory also allegedly lacked adequate hygienic facilities. Similarly, in its Toansa factory, FDA inspectors found flies. Wockhardt also faced similar troubles with hygiene.
Doubts about the quality of medicines made in India became stronger. Though many argued that the FDA's inspection parameters are different and far more stringent than those of other regulators, the incidents did raise concerns about the manufacturing practices and quality standards followed by Indian companies.
…

Though the FDA emphasised that not all Indian pharmaceutical companies were in the wrong, the atmosphere of suspicion created by these incidents overtook several other companies.
The repeated failure of Japanese major Daiichi Sankyo, the innovator parent of Ranbaxy, to restore compliance in the Ranbaxy factories, also raised doubts about India's seriousness about quality and safety measures.
Ensuring quality has never been easy in the gigantic Indian pharmaceutical industry. The sector, with over 20,000 registered pharmaceutical companies, remains inadequately regulated.
…

And this has been highlighted repeatedly in the past few years.
A 2010 report by International Policy Network found that 7 per cent of drugs purchased from wholesale traders were substandard, and 3.6 per cent of the drugs from retailers contained no active ingredients whatsoever.
Some of the spurious drugs contained chalk or talcum powder mixed with a pain reliever to trick and defraud the patient.
As many as 92 per cent of pharmacists said they had been offered substandard or spurious drugs for sale at cheaper prices.
…
Many companies, though not all, cut corners in order to protect their profit margins and glossed over processes to compress the go-to-market time.
The surge in the number of pharmaceutical companies came with the enactment of the Patent Act of 1970 which said India would respect only process patents.
This meant Indians were free to make any medicine in the world as long as they adopted a new process of manufacturing it.
Not only did this help to bring down the cost of medicines significantly, it also led to the indiscriminate mushrooming of pharmaceutical companies.
…

Before 1970, Indian companies like Cipla had lobbied hard for the change in patent laws because they had found their attempts to make medicines blocked by one patent or the other.
All they could do then was make old drugs that had gone off patent. Post 1970, for the next three decades or so, Indian pharmaceutical companies had a free run, until 2005, when India reverted to product patents.
All these years, Indian companies such as Cipla, Wockhardt, Dr Reddy's and Ranbaxy have been major suppliers of low-cost, generic medicines, not just for developing and developed markets, but also for emerging and underdeveloped markets.
…

Cipla, for example, has a sizeable market in Africa where it supplies affordable anti-retrovirals and other essential medicines.
Other countries like Brazil, Romania and Latin America are also hugely dependent on India-made, low-priced medicines.
Several domestic drug-makers maintain that the recent suspicions about indigenously manufactured medicines have been propagated by makers of expensive patented medicines.
…

The domestic companies, they say, have created market challenges for multinationals through fast product approvals and price competitions.
"They have become a huge threat to multinational companies which have, therefore, started strategising against them," says an observer.
Even so, the need for tighter regulation is being felt. To raise the regulatory standards in the sector, the industry has proposed that India should become a member of the Pharmaceutical Inspection Convention, an association of drug regulators who agree to follow certain standards. It is estimated that it will take India five years to raise its regulatory ecosystem to that level.
The concerns, however, require to be addressed sooner than that. The reputation and saleability of Indian drugs is, after all, at stake.