 | « Back to article | Print this article |
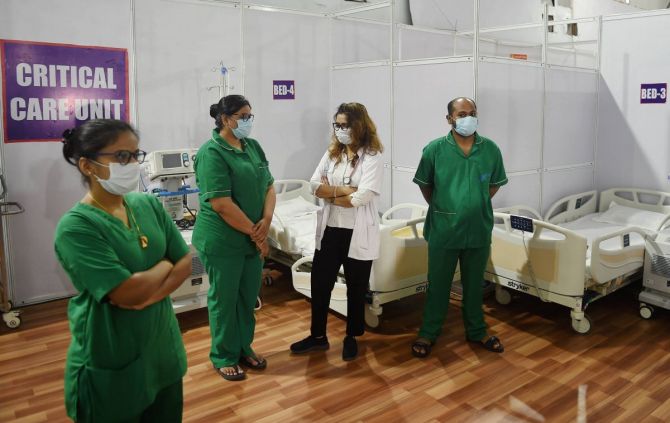
The second mock drill on COVID-19 vaccination was conducted on Friday in 736 districts across 33 states and union territories with Union Health Minister Harsh Vardhan, who oversaw the dry run in Tamil Nadu, saying inoculating the country's entire population would soon become a reality.

He said vaccines would be made available in the next few days and those at risk such as healthcare professionals would be given priority.
The Directorate General of Civil Aviation, on the other hand, issued guidelines to all aircraft operators who plan to transport COVID-19 vaccines packed in dry ice to various parts of the country.
If vaccines packed in dry ice are being transported in the passenger cabin of an aircraft, then the flight crew should be properly trained on the hazards and risks of its transportation, the aviation regulator noted.
Dry ice transforms into carbon dioxide gas at temperatures higher than -78 degrees Celsius under normal atmospheric pressure and, therefore, it is classified as "dangerous goods" by the International Civil Aviation Organization, it said.
The health ministry said the objective of the mock drill was to simulate the actual vaccine administration event. The entire planning of the vaccination drive including beneficiary registration, microplanning and vaccination at the planned session sites was tested under the leadership of district collector or district magistrate.
The dry run also aimed to familiarise the state, district, block and hospital level officers on all aspects of COVID-19 roll-out.

After reviewing the dry run at the Rajiv Gandhi Government General Hospital in Chennai, Vardhan said the Centre has started a new COVID platform to track particulars of potential vaccine beneficiaries and also issue electronic certificates to them.
India, he said, has done extremely well in developing vaccines in the shortest possible time.
"We are in the process of ensuring that in the next few days, also in the near future, we should be able to give this vaccines to our countrymen, starting with of course prioritising those who are most at risk, our health professionals, healthcare workers in the public and private sector followed by frontline workers," he added.
Vardhan also visited the Government Omandurar Hospital in Chennai and a few other centres.
He said the Integrated Vaccine Complex of HLL Biotech Limited, which has remained non-functional for six months, would soon be optimally utilised to produce COVID-19 vaccines.
The government has spent over Rs 600 crore to establish the state-of-the-art facility at Chengalpattu, he said.
HBL is a 100 per cent subsidiary of HLL Lifecare Limited, a government of India Enterprise under the ministry of health and family welfare.

Meanwhile, the Indian Medical Association has appealed to all its members to actively participate in the COVID-19 vaccination drive across the country.
"As you all are aware of the COVID-19 vaccine being made available in the immediate future, it becomes our natural responsibility to assist the vaccination drive in a professional way," the doctors' body said in a statement.
"With the vaccination against SARS-CoV-2 at our doorsteps, it is worth remembering that indigenous vaccines have been developed after the tireless efforts of Indian scientists in collaboration with the Indian Council of Medical Research and the National Institute of Virology," it said.
The first nationwide mock drill was held on January 2 which, the health ministry said, helped to iron out any glitches in the final execution and further refinement of the operational procedures.
India's drugs regulator has approved Oxford COVID-19 vaccine Covishield, being manufactured by the Serum Institute, and indigenously developed Covaxin of Bharat Biotech for restricted emergency use in the country.
Prime Minister Narendra Modi will interact with chief ministers of all states on Monday to discuss the COVID-19 situation and the vaccination roll-out in the country, the PMO said.
On Thursday, Vardhan interacted with the health ministers and principal secretaries and additional chief secretaries of all states and union territories through video conference to review the preparedness for the mock drill.
The drill was held at three session sites of 736 districts across 33 states and union territories.

In Maharashtra, where the drive was conducted in 32 of the 36 districts, Health Minister Rajesh Tope said there are still "some areas in the overall system where we need to improve efficiency in terms of data updating, delivery of SMS and training the local staff for inoculation".
Karnataka Health Minister K Sudhakar said the state is expected to receive 13,90,000 vials of COVID-19 vaccine in a day or two, and it is likely to be administered from January 11.
"We have registered 6.30 lakh healthcare professionals in Karnataka till date. Those who are left out, may be in some medical or dental colleges, we have requested them to register," he told reporters after visiting a private hospital in Bengaluru where the dry run was conducted.